The periodic table of elements is the most powerful tool for organizing chemical information. Without it, chemistry would be a chaotic, confusing jumble of seemingly random observations. What makes the periodic table really invaluable is. Its use as a predictive tool. In short, you can predict a lot about the chemical behavior of an element. Also, if you know where it is on the periodic table. Each element is represented by one square on the periodic table. With a one or two-letter chemical symbol. Many of the chemical symbols are derived from the English name for the element. But some come from other languages.
For example, the symbol for silver is Ag, from the Latin word argentum. Besides, the symbol for lead is Pb, from the Latin word plumbum. Above, the chemical symbol is the atomic number of the element. Below, the symbol are the full name of the element and its atomic mass.
Metals and Nonmetals in Periodic Table of Elements:
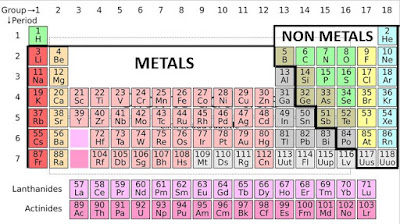 Most elements are metals. You can find them on the left and in the middle of the periodic table. In brief, metallic elements are typically shiny and are good conductors of heat and electricity. Besides, nonmetals are found on the upper right of the periodic table. Except for Hydrogen there on the left. It’s also a nonmetal. Nonmetals generally are NOT shiny and are NOT good conductors of heat or electricity.
Most elements are metals. You can find them on the left and in the middle of the periodic table. In brief, metallic elements are typically shiny and are good conductors of heat and electricity. Besides, nonmetals are found on the upper right of the periodic table. Except for Hydrogen there on the left. It’s also a nonmetal. Nonmetals generally are NOT shiny and are NOT good conductors of heat or electricity.The dividing line between metals and nonmetals on the periodic table is drawn as a thick staircase. The elements that are found on either side of that staircase are often called METALLOIDS. Also, they have properties that fall between metals and nonmetals. In brief they are Boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At). So, notice that the atoms are listed in order of increasing atomic number. As you read the periodic table from left to right, top to bottom. Each element has a unique atomic number. That’s the number of protons in the nucleus of the atom.
Rows and Columns in Periodic Table:
So, why are the elements organized into rows and columns? Why don’t we just put the elements in a long list? It turns out, if you arrange elements by atomic number, a pattern emerges. Also, there is a periodicity or a repeating of certain characteristics. For example, every so often, an inert gas appears. Right next to it will be an element that reacts violently with water. This periodic repetition is known as the PERIODIC LAW. So the, this is the basis for organizing the elements in the Periodic Table into columns.
Vertical Column:
A vertical column of elements is called a GROUP or a FAMILY. The elements in a group have similar chemical properties. Here, we now know that’s because they have similar valence electron configurations.
Horizontal Rows:
There are 7 horizontal rows in the periodic table. These rows are called PERIODS. Each row corresponds to a different energy level occupied by electrons.
Alkali Metals and The Alkali Earth Metal:
 The two groups on the left are the alkali metals and the alkali earth metal. The s orbitals in the outermost shell of the atom are being filled in these groups.
The two groups on the left are the alkali metals and the alkali earth metal. The s orbitals in the outermost shell of the atom are being filled in these groups.There are six Alkaline metals in group 1 of periodic table. Names are Lithium (Li), Sodium (Na), potassium (k), Rubidium (Rb), Cesium (Cs), & Francium (Fr) are alkali metals. Because they react with water.
In addition, there are six Alkaline earth metals in group 2 of periodic table. Names are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) & Radium (Ra). These Alkaline earth metals are all shiny silvery white
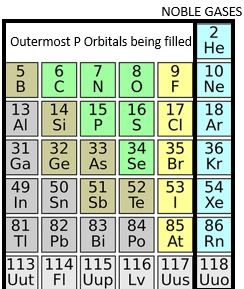
Noble Gases:
On the right is a block of 6 columns. These elements have the outermost p orbitals being filled. Notice on the far right are the Noble gases. Which all have a filled valence shell of electrons. There are six noble gases that occur naturally. So, they are helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn).
Transition Metals:
In the middle is a block of 10 columns. The transition metals. So, in these elements, the outermost d orbitals are being filled.
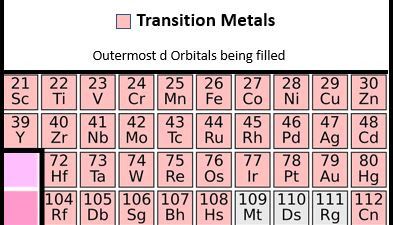
In addition, there are 40 transition metals. They are Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium, Rhodium, Palladium. Silver, Cadmium, Lutetium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury, Lawrencium, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium. Roentgenium, and Copernicium.
Inner Transition Metals:
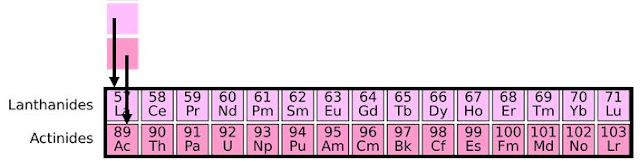
The asterisks take you to the bottom of the Periodic Table. where there are two rows of 14 columns. The inner transition metals. Also, known as the Lanthanides and Actinides. These elements have the outermost f orbitals being filled.
Lanthanides:
The period 6 inner transition metals lanthanides are cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu). gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu).
Actinides:
In detail, the period 7 inner transition metals actinides are thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), americium (Am). curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No), and lawrencium (Lr).
Finally, this is a brief explanation of periodic table and its elements.