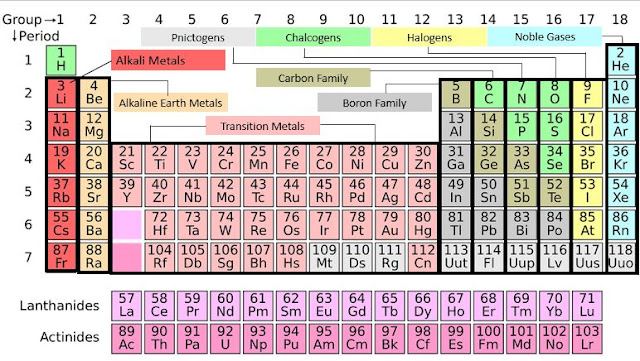
Periodic table groups are also
known as chemical families. These can be found as columns in the periodic
table. The key thing to remember with groups or chemical families is that the
elements within a same column. They are gonna have similar chemical and
physical properties. So, that means lithium, sodium potassium, rubidium, cesium
and francium are all gonna have very similar chemical and physical properties. So,
they're gonna kind of look and act the same.
Periodic Table Groups Explanation
What's interesting about the
periodic table groups? In brief, all of the elements in a column for the most
part and there's tons of exceptions. But for the most part the elements in the
column have very similar properties. That's because the elements in a column or
the elements in a group. In short, they tend to have the same number of valence
electrons and electrons in the outermost shell. They tend to coincide.
Although, there's a slightly different variation. Furthermore, the valence
electrons. These are the electrons that are going to react, which tend to be
the outermost shell electrons.
Group 1 Alkali Metals - Periodic Table Groups

- They are very reactive elements.
- They have one valence electron which makes them react.
- They're going to be a very soft metal.
- They have low melting points and boiling points.
- They're gonna be found in compounds reacted with halogens.
Group 2 Alkaline Earth Metals - Periodic Table
Groups
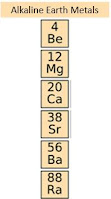
- They're fairly reactive but less reactive than the alkali metals.
- They're gonna be shiny much harder than the alkali metals.
- They are silvery and white color.
- They're gonna have two valence electrons.
- The alkaline earth metals are going to be a solid at room temperature.
Groups 3 to 12 Transition Metals - Periodic
Table Groups

Scandium (Sc), Titanium (Ti),
Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel
(Ni), Copper (Cu). Zinc (Zn), Yttrium (Y), Zirconium (Zr), Niobium (Nb),
Molybdenum (Mo), Technetium (Tc), Ruthenium (Ru), Rhodium (Rh), Palladium (Pd).
Silver (Ag), Cadmium (Cd), Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium
(Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au). Mercury (Hg),
Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrium (Bh), Hassium (Hs),
Meitnerium (Mt), Darmstadtium (Ds), Roentgenium (Rg), Copernicium (Cn).
Group 13 Triels or Boron Family
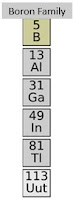
- They do not occur elementally in nature.
- They're pretty scarce except for aluminum which is one of the most abundant metals in our universe.
- Boron family have three valence electrons.
- They are metallic except for boron which is the Metalloids.
- They are also chemically reactive at moderate temperatures except for boron.
Group 14 Tetragens or Carbon Family

- In the carbon family, has one nonmetal which is carbon.
- They have two metals, tin and lead.
- They have two metalloid, it's silicon and germanium
- Carbon family have four valence electrons.
- They tend to form covalent bonds.
- They have gas ability to combine with itself and long chains.
- It also exists in at least three allotropic forms.
- Graphite and diamond.
Group 15 Pnictogens or Nitrogen Family

- In the nitrogen family, has two nonmetal which is nitrogen and phosphorus.
- They have one metal, its bismuth.
- They have two metalloid, it's arsenic and antimony.
- Nitrogen Family have five valence electrons and so in knowing that we know that
- They form covalent compounds with oxidation numbers of +3 or +5.
Group 16 Chalcogens or Oxygen Family
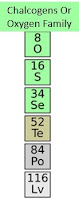
- The oxygen family has six valence electrons.
- Oxygen tends to form covalent compounds with other elements.
- The two most common molecular forms of O2 and O3.
- O2 is oxygen. O3 is ozone gas. They are allotropes. There are different forms of the same element in the same state.
- Also, oxygen has a tendency to attract electrons from other elements.
- Oxygen in combination with metal is almost always present as the oxide ion or O2 negative.
- Sulfur also exists in several allotropic forms. One of the most common is s8.
- Also, sulfur gain electrons to form sulfides that contain the s2 negative ion.
Group 17 Halogens or Fluorine Family

Group 18 Noble Gases or Helium/Neon Family
Conclusion of Periodic Table Groups
So, that's a brief introduction to periodic table groups and its key points are.
- Group 1 has 06 elements and is called alkali metals or lithium family.
- Also, Group 2 has 06 elements and is called alkaline earth metals or beryllium family.
- Groups 3 to 12 has 38 elements and is called transition metals.
- Group 13 has 06 elements and is called triels or boron family.
- Also, Group 14 has 06 elements and is called tetrels/tetragens or carbon family.
- Periodic table Group 15 has 05 elements and is called pnictogens or nitrogen family.
- Group 16 has 06 elements and is called chalcogens or oxygen family.
- Group 17 has 06 elements and is called halogens or fluorine family.
- Lastly, Group 18 has 07 elements and is called noble gases or helium/neon family.